
In action Jan. 19, Secretary of Agriculture Sonny Perdue signed a Memorandum of Understanding with the Assistant Secretary for Health and Head of the Public Health Service Admiral Brett Giroir, M.D., establishing a regulatory environment at USDA for agricultural animal biotechnology innovation. However, Food and Drug Administration Commissioner Dr. Stephen Hahn says despite the MOU, FDA will not hand over oversight to USDA.
“USDA and FDA have a long history of delineating the review of products with overlapping jurisdictional authority between the two agencies to promote regulatory clarity and reduce duplicative review,” USDA states. However, Hahn continues to say human health oversight should remain at FDA. The back and forth between USDA and FDA culminates over a year of discussion between Perdue’s comments and actions at USDA and FDA’s staunch desire to see the oversight remain under their jurisdiction.
Agricultural biotechnology holds tremendous potential to improve animal health, enhance farm productivity, improve nutrition and even reduce the need for some animal health measures.
“In the past, regulations stifled innovation, causing American businesses to play catch-up and cede market share,” says Perdue. “America has the safest and most affordable food supply in the entire world thanks to the innovation of our farmers, ranchers and producers. Establishing a new, transparent, risk and science-based regulatory framework would ensure this continues to be the case.”
Related: FDA, USDA still at odds regarding livestock gene-editing
The MOU complements USDA’s issuance of an Advanced Notice of Proposed Rulemaking on the Movement of Animals Modified or Developed by Genetic Engineering on December 28, 2020.
The terms of the MOU support USDA’s ANPR outlining a contemplated regulatory framework that would apply to certain animals (cattle, sheep, goats, swine, horses, mules, or other equines, catfish, and poultry) developed using genetic engineering intended for agricultural purposes. Under this framework, USDA would safeguard animal and human health by providing end-to-end oversight from pre-market reviews through post-market food safety monitoring for certain farm animals modified or developed using genetic engineering that are intended for human food.
The MOU also allows for the transition of portions of FDA’s pre-existing animal biotechnology regulatory oversight to USDA. USDA would continue to coordinate closely with FDA to fulfill oversight responsibilities and provide the appropriate regulatory environment, ensuring the safety of products derived from new technologies and fostering innovation at the same time. A statement from USDA says as always, FDA would continue its review of intentional genomic alterations intended for any purpose other than agricultural use, such as biopharma and non-heritable genomic alteration, and the regulation of dairy products, table and shell eggs, certain meat products, and animal food (feed) derived from animals developed using genetic engineering.
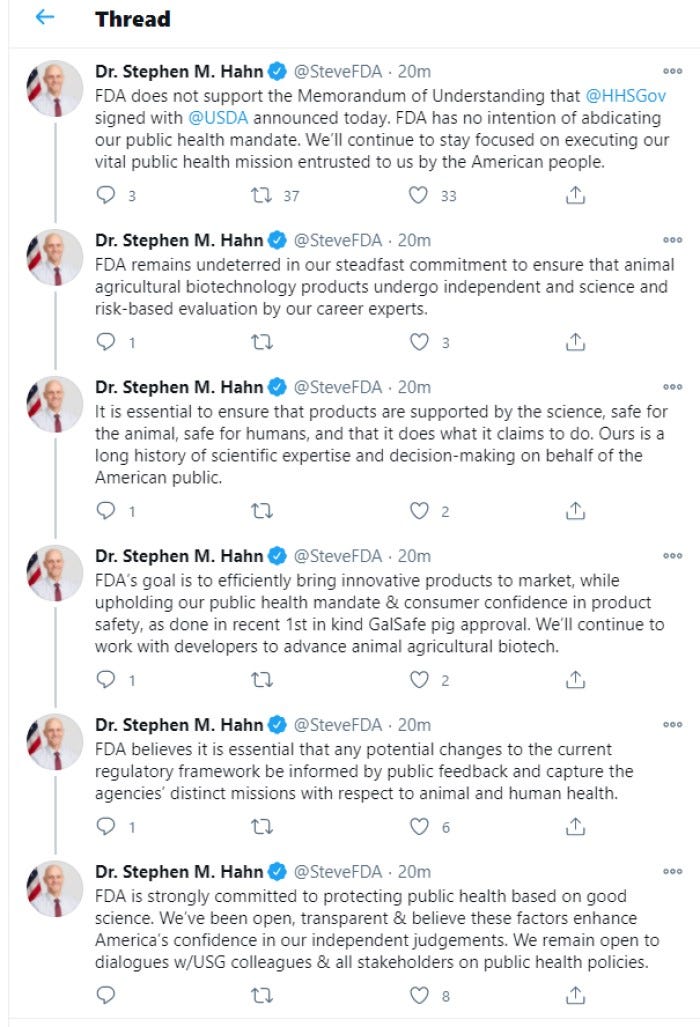
In a series of tweets from Hahn, he says “FDA has no intention of abdicating our public health mandate….FDA’s goal is to efficiently bring innovative products to market, while upholding our public health mandate & consumer confidence in product safety, as done in recent 1st in kind GalSafe pig approval. We’ll continue to work with developers to advance animal agricultural biotech.”
Groups respond to USDA oversight
Agricultural and biotechnology groups have been supportive of the transition of the oversight to USDA.
“This is a promising step. Oversight of animal biotechnology needs to be clear and workable for technology developers in order to maintain America’s position as a global leader in agricultural innovation,” says Karen Batra, communications manager at BIO. “We appreciate this initiative, and BIO looks forward to working with the incoming administration on efforts to implement an updated regulatory framework.”
The National Pork Producers Council praised the MOU. NPPC has been an outspoken proponent urging the jurisdiction transition giving USDA primary regulatory jurisdiction over the development of gene-edited livestock.
“NPPC has been calling for this decision for more than three years to ensure that U.S. agriculture maintains its competitive edge globally. We look forward to working with the Biden administration to implement a technology that has the potential to improve animal health, further reduce agriculture’s environmental footprint and improve production efficiency,” NPPC says.
Kevin Scott, soy grower from Valley Springs, South Dakota, and American Soybean Association president adds, “Genetic solutions have the capability of protecting the health of our herds and flocks, and ASA continues to support predictable, timely, science-based processes for making these innovations available to producers. We have great confidence in USDA’s ability to develop an appropriate regulatory pathway for these important tools.”
About the Author(s)
You May Also Like






