May 10, 2017

I recently had a discussion in the middle of a corn field about water pH and hardness with a very astute grower from middle Georgia. As I recall, this was also a common topic during my 2017 winter meeting season. Although pH and hardness are two totally different things, both can have an influence on the performance of herbicides if they are out of whack.
Water pH is a measure of the hydrogen (H+) and hydroxide (OH-) ions in a solution. Water with a pH value of 7.0 is considered to be neutral. Water with a pH below that is considered to be acidic (<7.0) and above that, alkaline (>7.0). Keep in mind though that these values are based on a logarithmic scale so a pH of 6.0 is 10 times more acidic than 7.0 and 100 times more acidic than 8.0.
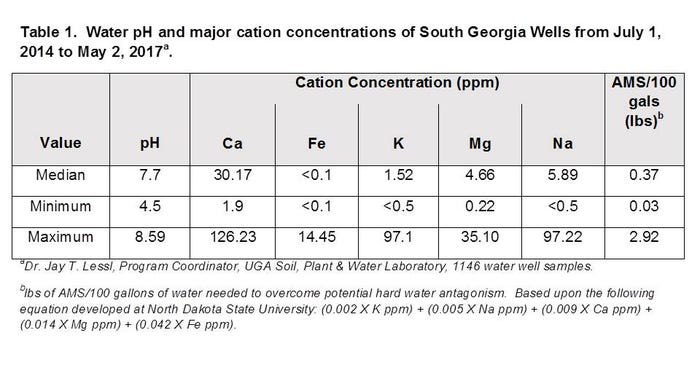
Water pH is important for many herbicides because they can be broken down or degraded via a process known as alkaline hydrolysis when the pH is too high. For most herbicides, a pH range between 4.5 and 7.0 is fine. Get above 7.0 and you need to consider adding something (i.e. buffer, water conditioner, etc.) to lower that number. Your local pesticide purveyor would be more than willing and able to provide you with their favorite. Check out Table 1 for some recent Georgia water pH values from Dr. Jay Lessl at the UGA Soil, Plant & Water Lab.
Water hardness, at least in my simple and fading mind, is a measure of the various cations that can be found in water. The most important of these cations are calcium (Ca), iron (Fe), potassium (K), magnesium (Mg), and sodium (Na). Since these cations have a positive (+) charge, they can bind with certain negatively (-) charged herbicides thus forming complexes that are more difficult to move into a plant. This phenomenon is often referred to as hard water antagonism.
Hard water antagonism is the primary reason that ammonium sulfate (AMS) adjuvants are recommended when using glyphosate. AMS adjuvants may also help maintain a more acidic pH. Not all herbicides are affected by hard water antagonism. Cation concentrations from current samples of South Georgia wells are also listed in Table 1.
I know that you might get tired of hearing this, but the herbicide label is a great source of information for all types of goodies, including the need or not for adjuvants and water conditioners. When in doubt, check those labels out! Great late-night reading (not)!
Now the only way that you can really know what your water pH and cation concentration levels are is by periodically having your water tested. The UGA Soil, Plant & Water Lab will conduct a simple water test for about $20 (http://aesl.ces.uga.edu). You could also purchase an inexpensive (<$100) pocket pH meter as well. Either way, that is a small price to pay for getting very valuable information. To quote a popular TV commercial for older folks like me, “Do you know your numbers?”
It seems as though collecting a simple water sample is primitive in comparison to all of the modern technologies that growers have at their fingertips. However, understanding and knowing your water pH and cation concentrations might help you save money when adjuvants/conditioners are not needed or help you get better weed control if they are.
As always, good weed hunting!
About the Author(s)
You May Also Like






