February 1, 2023

Soil is comprised of organic matter, minerals, water, air, and organisms on the Earth’s surface that enable farmers to plant, manage, and harvest high-yielding crops every year. Soil is the medium that allows seeds to germinate, emerge, take root, and develop into plants. Productive soil has appropriate soil tilth, drainage, microbial activity, and nutrient availability to support plant growth.
Nutrient availability is mainly dependent on soil pH, which affects the solubility of nutrients and the amount of plant nutrients available in the soil solution, thereby affecting nutrient availability. Fourteen of the 17 essential elements for plant growth are derived from the soil. Chlorine and nickel are relatively unaffected by soil pH, and carbon, hydrogen, and oxygen are obtained from the air and water.
pH is an abbreviation for the potential (p) for hydrogen ions (H+) in water and is presented on a 14-point logarithmic scale (a pH of 6 is 10 times more acidic than pH of 7). A value of 7.0 is neutral, while values lower than 7.0 indicate an increase in the H+ ion concentration and acidity. Values higher than 7.0 increase the hydroxyl (OH-) ion concentration and alkalinity. Alkaline soils are also called basic soils. The pH is determined through soil sampling. Most soils in Midwest fields become acidic over time through environmental and farming practices.
A soil pH level is a component of all standard soil sample tests and measures hydrogen ion (H+) and hydroxyl ion (OH-) concentration. Soil pH is a 14-point scale, with 7.0 being neutral. As the amount of H+ ions in the soil increases, the soil becomes more acidic (pH <7).
As the amount of OH- ions in the soil increases, the soil reaches a more alkaline ph (pH>7). Soil pH is dependent on many factors, including parent material, rainfall, and even agricultural practices. What does this have to do with nutrient availability? The answer is… a lot.
Our soil pH greatly impacts the solubility of minerals or nutrients and, therefore, the availability of plant uptake. Restricted root growth can compound the problem as the plant is unable to reach enough of the soil profile to compensate for reduced nutrient availability. Commercial fertilizers and natural soil biology will not be as effective if the pH is not managed. Figure 1 highlights the influence of pH on soil nutrient availability. The optimal pH for plant growth is dependent on the crop, but the best soil pH for corn and soybeans would be between 6.0 to 6.5.
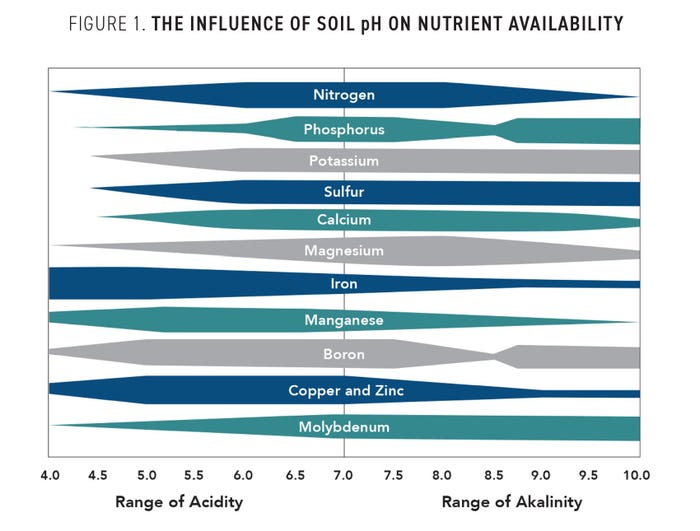
Photo Submitted by Beck's Hybrids
ACIDIC SOILS
In acidic soils with a pH less than 6.0, the availability of nitrogen (N), phosphorus (P), potassium (K), sulfur (S), calcium (Ca), and other nutrients is reduced. Not only are these nutrients chemically less available at lower pH levels, but the microbial activity is diminished. This can slow down soil processes like the nitrogen cycle.
The availability of nutrients like iron (Fe), manganese (Mn), copper (Cu), zinc (Zn), and aluminum (Al) increases in acidic soils. But the increases could be at levels that are toxic to the plant, especially in the case of Al, Fe, and Mn.
CORRECTING ACIDIC SOILS
The good news is that acid soils can be corrected by applying lime. A good agricultural liming material neutralizes soil acidity with the help of Ca and/or Mg by replacing H+ ions and, consequently, raising the soil pH. Not all lime is created equal; most soil testing labs will analyze lime to determine its neutralizing value.
Growers may start to see benefits from lime during the first few months after application, but it will take two to three years for it to react completely with the soil. According to Michigan State University, increasing your soil pH from 5.7 to 6.5 in mineral soils can improve corn and soybean yields by 20% or more.
ALKALINE SOILS
High pH soils often have a reduced availability of P, Zn, Fe, Cu, Boron (B), and Mn, which results in stunted plant growth. In general, as pH rises, most micronutrients decrease in availability apart from molybdenum. Alkaline soils typically have higher levels of Ca, Mg, and sodium (Na). The majority of high-pH soils contain calcareous materials such as limestone and are often found in arid crop areas like the Great Plains.
CORRECTING ALKALINE SOILS
Unfortunately, correcting soils with acidity or alkalinity is no easy task. Soil pH can be reduced by applying elemental S, but it's generally not economically feasible.
The best option may be adding organic matter via manure applications or increased crop residue. However, this can take years to impact pH and, subsequently, crop performance. It is best to focus on hybrid and variety selection with good tolerance to high pH soils.
For more information on soil nutrients and pH, click here.
Beck's - Farmers At Heart® - revolutionized the customer seed buying experience by remaining true to a foundation built on faith, family, and farming. Founded in 1937, Beck's appreciates the farmers who have helped them become the largest family-owned retail seed company and the third largest seed brand in the United States. The Beck family is now in its fifth generation of family members who work in the business to honor God and help farmers succeed. The Beck family and team of employees help farmers achieve success from generation to generation through authentic customer experiences, product diversity, seed quality, and performance. With a home office located in Atlanta, Ind., Beck's serves farmers throughout the Midwest and Mid-South. For more information about Beck's Superior Hybrids, Inc., visit www.beckshybrids.com.
You May Also Like





