The U.S. Food and Drug Administration approved a first-of-its-kind intentional genomic alteration (IGA) in a line of domestic pigs, referred to as GalSafe pigs, which may be used for food or human therapeutics. This is the first IGA in an animal that the FDA has approved for both human food consumption and as a source for potential therapeutic uses.
The IGA in GalSafe pigs is intended to eliminate alpha-gal sugar on the surface of the pigs’ cells. People with Alpha-gal syndrome (AGS) may have mild to severe allergic reactions to alpha-gal sugar found in red meat (e.g., beef, pork, and lamb).
“Today’s first ever approval of an animal biotechnology product for both food and as a potential source for biomedical use represents a tremendous milestone for scientific innovation,” says FDA Commissioner Stephen M. Hahn, M.D. “As part of our public health mission, the FDA strongly supports advancing innovative animal biotechnology products that are safe for animals, safe for people and achieve their intended results.”
Clint Nesbitt, senior director of science and regulatory affairs at BIO, states, “This is another milestone for animal biotechnology and illustrates the potential genetic engineering holds to enhance our food and improve public health. Thanks to biotechnology, companies are developing breakthroughs that can further benefit society and we must continue to develop a modern and transparent regulatory system that allows for more of these innovations to be realized.”
Turf war
However, the action by FDA also reiterates the ongoing disagreement from those in the agriculture industry desiring USDA to have authority over animal biotechnology approvals, rather than FDA. In more than two decades, only one food animal has been approved by the FDA’s biotechnology process.
National Pork Producers Council Director of Science and Technology Dr. Dan Kovich says, however, “NPPC remains firm that, in order for the tremendous potential of gene editing to be safely and readily available to U.S. farmers, regulatory authority for all agricultural applications of these new technologies has to be consolidated at the USDA.”
Hahn says the action “underscores the success of the FDA in modernizing our scientific processes to optimize a risk-based approach that advances cutting-edge innovations in which consumers can have confidence.”
“The FDA is committed to continuing its close work with developers to facilitate safe advancements of animal biotechnology. Our Veterinary Innovation Program focuses on providing greater certainty in the regulatory process, encouraging development and research of innovative public health products, as well as supporting an efficient and predictable pathway to the approval of IGAs in animals,” adds Steven M. Solomon, D.V.M., M.P.H., director of the FDA’s Center for Veterinary Medicine.
Medical use
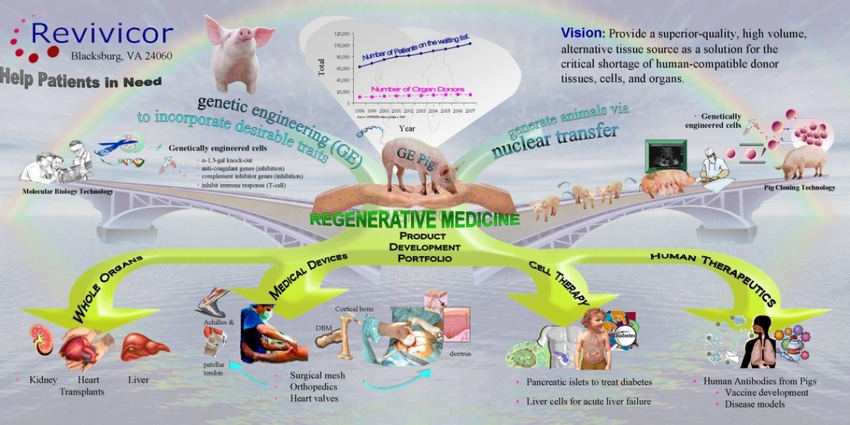
Dewey Steadman, head of investor relations at United Therapeutics Corporation, says Revivicor and United Therapeutics are excited to gain approval for the first-of-its-kind intentional genomic alteration in a line of domestic pigs.
“This advancement, which eliminates the presence of alpha-gal sugar in porcine products raised with the alteration, further validates the potential for future development of porcine products in a number of areas where the presence of alpha-gal sugar could cause significant reactions in patients with alpha-gal syndrome,” Steadman says. “We look forward to working with potential partners in food, drug, and medical device production to potentially bring these products to market.”
Steadman notes, “This gene edit is just one of ten edits we are currently using in pigs for our preclinical xenotransplantation development program that we hope one day will help address the critical shortage of transplantable organs for humans in need.”
GalSafe pigs may potentially provide a source of porcine-based materials to produce human medical products that are free of detectable alpha-gal sugar. For example, GalSafe pigs could potentially be used as a source of medical products, such as the blood-thinning drug heparin, free of detectable alpha-gal sugar. Tissues and organs from GalSafe pigs could potentially address the issue of immune rejection in patients receiving xenotransplants, as alpha-gal sugar is believed to be a cause of rejection in patients. Solomon adds that alpha-gal syndrome results from individuals being bitten by a specific species of ticks, and an increasing number of the population is impacted by it.
As part of its review, the FDA evaluated the safety of the IGA for the animals and people eating meat from them, as well as the product developer’s intention to market the IGA for its ability to eliminate alpha-gal sugar on pigs’ cells. The FDA determined that food from GalSafe pigs is safe for the general population to eat. Although FDA approved the animals for food use, Solomon says this line of hogs are not expected to be in the food supply chain.
The FDA’s review also focused on ensuring the effectiveness of the IGA through the evaluation of data demonstrating that there is no detectable level of alpha-gal sugar across multiple generations of GalSafe pigs.
About the Author(s)
You May Also Like