Numerous businesses across the country are working on providing consumers with more choices for protein sources. The U.S. Food and Drug Administration recently made two announcements that could bring new protein options one step closer to the market.
Food from cultured chicken cells
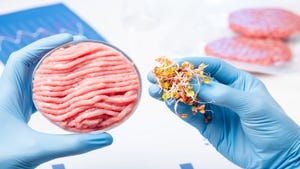
California startup UPSIDE Foods received the green light from the FDA on its cultivated chicken product. The food product is made by harvesting cells from chickens and growing the cells in a controlled environment to make cultured animal cell food.
The FDA issued a letter to UPSIDE Foods on Nov. 16 after completing its first pre-market consultation, which has been in the works since fall of 2021.
“We evaluated the information UPSIDE Foods submitted to the agency and have no further questions at this time about the firm’s safety conclusion,” FDA states.
The letter indicates that the FDA accepts UPSIDE’s conclusion that its cultivated meat product is safe to eat – however, the voluntary pre-market consultation is not an approval process.
The food still requires a mark of inspection from USDA-FSIS before it can enter the U.S. market. “As this product comes closer to entering the U.S. market, we are closely coordinating with USDA-FSIS to ensure it is properly regulated and labeled,” FDA wrote in a statement.
UPSIDE Foods is the first cultivated meat company to reach this milestone in the U.S.
Public hearing on AquAdvantage Salmon
On Nov. 16, the FDA issued an updated environmental assessment for AquAdvantage Salmon, a genetically modified Atlantic salmon developed by AquaBounty Technologies that grows quicker than conventional, farm-raised Atlantic salmon.
In 2015, the FDA approved the application of AquAdvantage Salmon. FDA’s environmental assessment concluded that AAS would not jeopardize U.S. populations of Atlantic salmon or result in harm of their habitat and issued its Environmental Assessment and Finding of No Significant Impact at the time of approval.
Subsequently, several organizations filed suit challenging the FDA’s evaluations for the 2015 approval. On Nov. 15, 2020, the Court ordered the FDA to do additional analysis and reconsider its “no effect” determination. The amended draft recently issued addresses the Court’s order.
The FDA is seeking public comment on the draft amended EA through Jan. 17, 2023 and will hold a virtual public meeting on Dec. 15. Visit FDA’s meeting webpage for more information.
Read more about:
Food ProductionAbout the Author(s)
You May Also Like